UNDERSTANDING HOW FAILURE OF THE PROTEIN QUALITY CONTROL LEADS TO AGING AND AGE-RELATED NEURODEGENERATIVE AND NEUROMUSCULAR DISEASES
The formation of biomolecular condensates by phase separation has emerged as a new way of organizing the cytoplasm and nucleoplasm of cells. The main components of these condensates are RNAs and proteins. In order to assemble by phase separation, proteins and RNAs undergo cooperative interactions that are supported by specific molecular features, such as multivalency and disordered domains. However, these features also predispose phase-separating proteins to aberrant behaviour and aggregation. Our lab was a pioneer in showing an unexpected link between the protein quality control system and condensate dynamics. The main focus of my research is to understand the role of molecular chaperones in regulating the properties and functions of biomolecular condensates, thus enabling dynamic cellular compartmentalization in response to physiological and external stimuli on the one hand, and preventing age-related neurodegenerative and neuromuscular diseases, on the other hand.
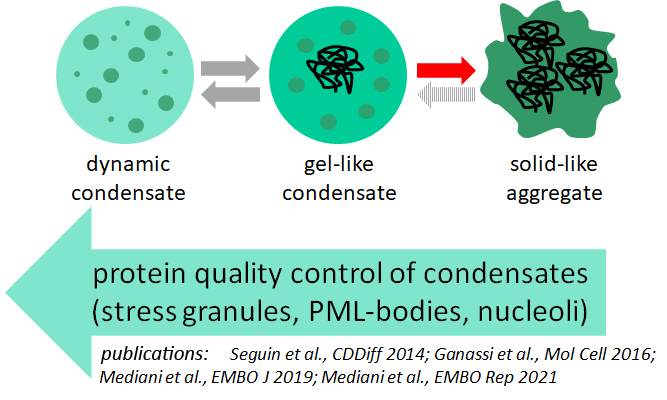
My lab is focusing on two main research lines. First, using cellular models and depleting or inhibiting specific players of the protein quality control system, we aim at understanding the role of molecular chaperones in regulating the properties and functions of biomolecular condensates in response to physiological and external stimuli, with the ultimate goal of identifying key factors that may help preventing irreversible aggregation.
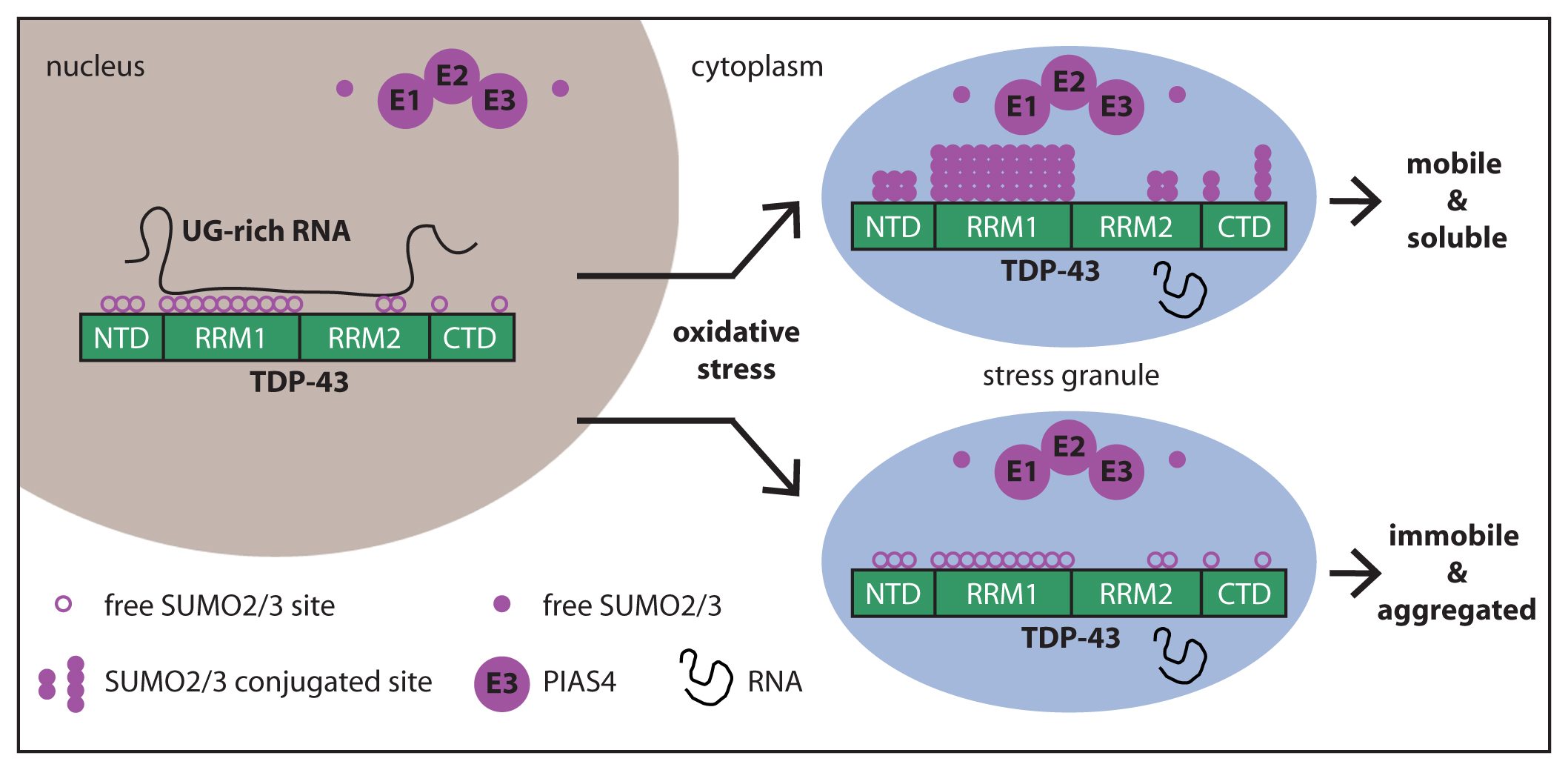
When cells are not subjected to stress, TDP-43 is mainly localized inside the nucleus, where it binds with high affinity to UG-rich RNA. RNA masks the K residues located in the RRM1 and RRM2, reducing the access to PIAS4. Upon oxidative stress, a fraction of TDP-43 translocates into the cytoplasm, where it localizes inside SGs along with the SUMO machinery (free SUMO2/3, E1, E2 and E3 enzymes, including PIAS4). RNA-free TDP-43 molecules can be efficiently conjugated to SUMO2/3 by PIAS4, thereby protecting TDP-43 from immobilization inside SGs and aggregation.
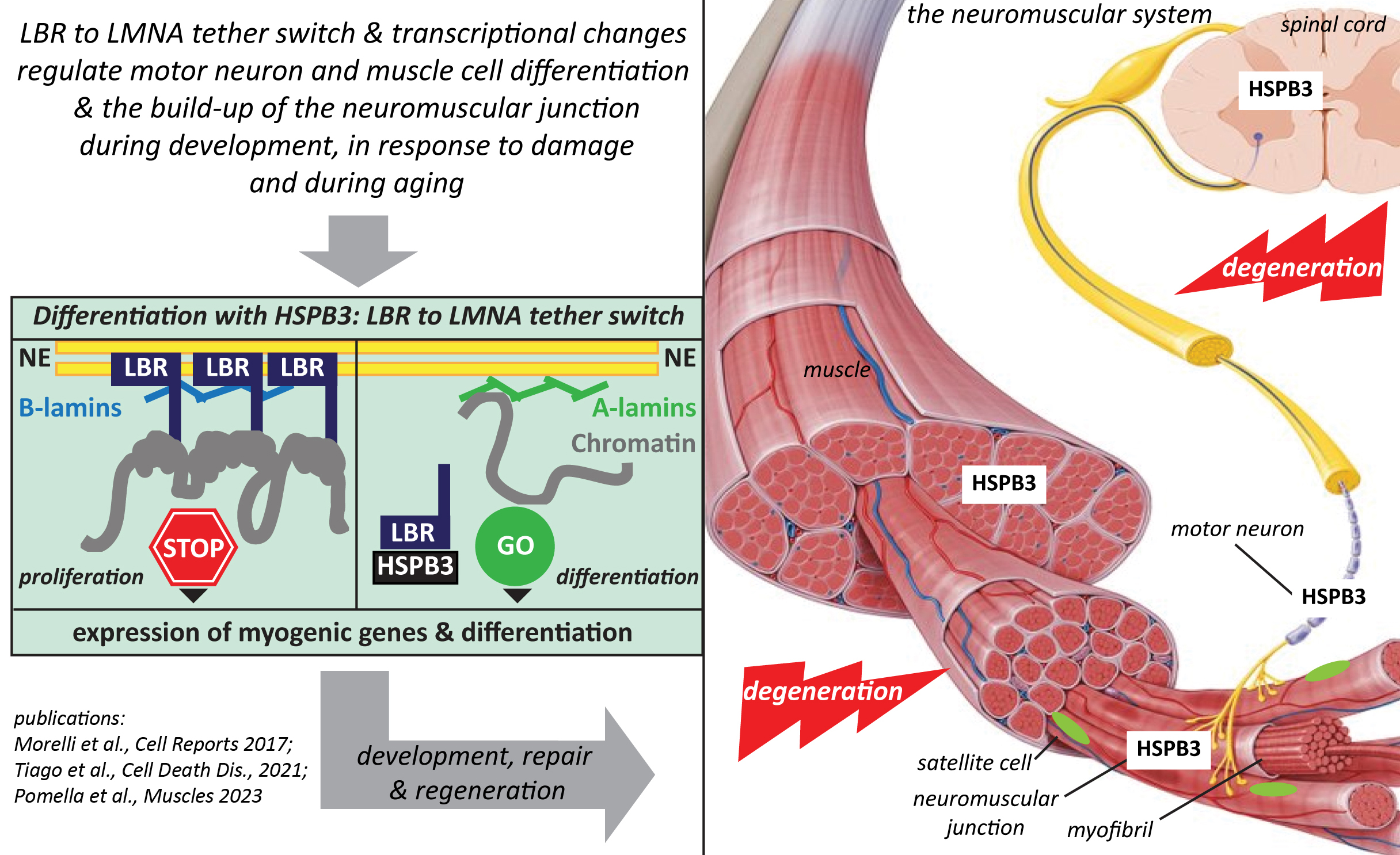
Second, chaperones themselves can form/are recruited into biomolecular condensates, which may help explaining their implication in complex processes such as transcriptional regulation and cell differentiation. Using iPSC-derived motor neurons and skeletal muscle cells, we’re studying the role of HSPB3 and its disease-associated variants in the differentiation of these two cell types, with the ultimate goal of unravelling HSPB3 implication in the maintenance of the neuromuscular system during aging and its association with motor neuropathies and myopathies.
–